Answer:
Welcome, students, parents or teachers in this article we are going to give the answer of What are canal rays? Take a note that in exams you can add more points in answer to score more. To earn extra marks you can explain it in about 300 words. Thanks for reading the answer.
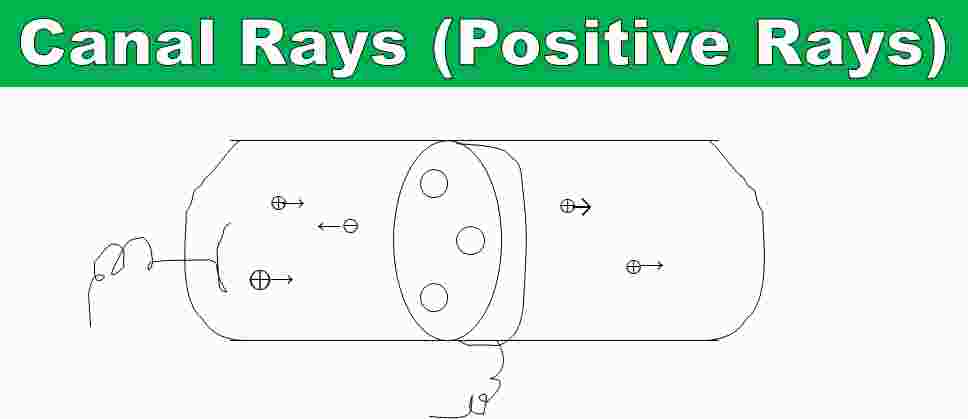
What are canal rays?
Canal rays, also known as cathode rays, are streams of negatively charged particles that are produced in a vacuum tube. These particles are produced when an electric potential is applied to the cathode, which causes electrons to be emitted. Canal rays were first discovered by J.J. Thomson in 1897 and were used as the basis for the early experiments that led to the discovery of the electron.
In addition to their historical significance, canal rays have also been used for a variety of applications, including X-ray tubes, cathode ray tubes in televisions and computer monitors, and cathode ray oscilloscopes for measuring electrical signals. The nature of canal rays was a subject of much debate in the early 20th century, but they are now understood to be composed of electrons.
What is the difference between canal rays and proton?
Canal rays and protons are two different types of particles.
Canal rays are beams of positive ions that are generated in a vacuum tube when a high voltage is applied to electrodes. They were first observed by J.J. Thomson in the late 19th century and were used in early experiments to study the properties of cathode rays.
Protons, on the other hand, are subatomic particles that make up the nucleus of an atom. They are positively charged and are much heavier than electrons. Protons are the building blocks of atomic nuclei and are responsible for determining the atomic number of an element.
In summary, canal rays are beams of positive ions while protons are subatomic particles that make up the nucleus of an atom.
Also Read: What is shifting cultivation what are its 5 disadvantages?