Understand The Relation Between Surface Tension and Surface Energy
Discover the complex relation between surface tension and surface energy as you delve into the fascinating world of intermolecular forces and learn about the subtle dynamics that control liquid interfaces and material behavior.
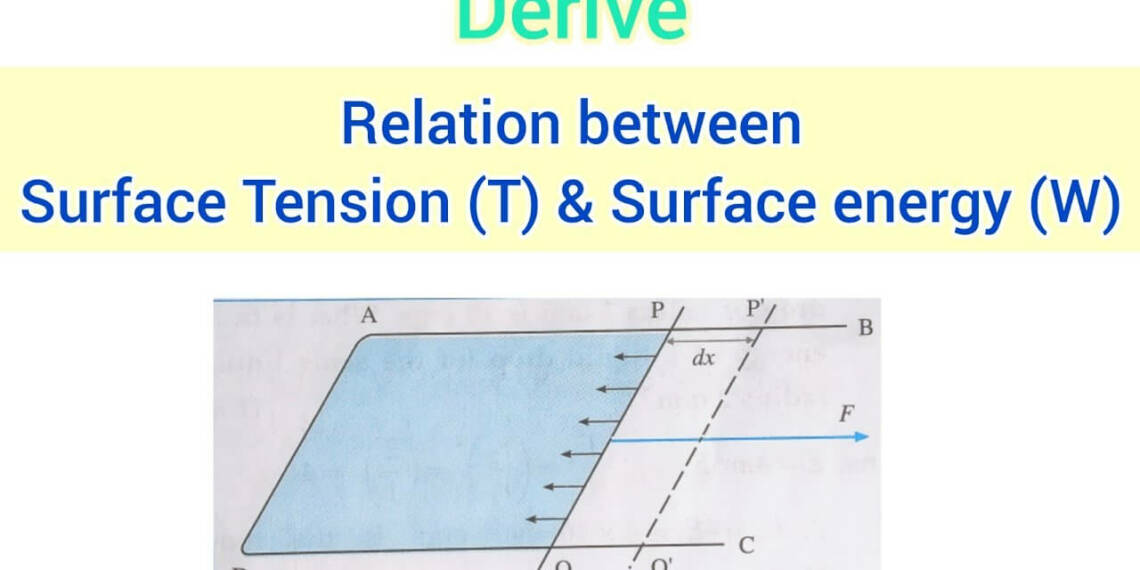
Relation Between Surface Tension and Surface Energy
Assume you are holding a cup of water. Take a close look at the water’s surface, which is the topmost layer where the liquid and air meet. This surface acts as a kind of skin that holds the molecules of water together, not just as a boundary. We refer to this skin-like characteristic as surface tension.
Now consider surface energy as this surface’s hidden force. It’s the energy needed to form and preserve that water-based skin. That’s basically the ‘cost’ of maintaining the tightly knit structure of the water molecules at the surface.
So, What is the relation between surface tension and surface energy
It turns out that surface tension and surface energy are essentially the same thing. Surface energy leads to surface tension. It would be equivalent to stating that the amount of work you put into tying your shoelaces (surface energy) is directly correlated with their tightness (surface tension).
Imagine this: Surface tension would vary as well if you could alter the water’s surface energy with a magic wand. Higher surface tension results from the water’s skin tightening as surface energy rises. Conversely, lowering surface energy causes the skin to become more flexible, which lowers surface tension.
Also Read: Write A Newspaper Report on the Jallianwala Bagh Massacre
Now, why does this matter?
Consider insects that appear to be able to “walk on water.” This relationship keeps their little legs from breaking the surface of the water. Their ability to stay atop is due to surface tension, which is fueled by surface energy.
Surface tension and surface energy affect various aspects of daily life, such as the way liquid soap spreads on water, the form of raindrops, and even the formation of bubbles.
Surface energy is the energy required to keep the surface tight, whereas surface tension is similar to the tightness of the surface. You can alter one by altering the other. Comprehending this correlation benefits scientists and engineers across multiple domains, ranging from devising enhanced products to deciphering the secrets of the natural world. It is comparable to removing the layers from a basic water surface to reveal a vast network of interrelated forces below.